The high price of new drugs has become a political hot potato. Hillary Clinton made drug affordability a part of her platform -- as did President-elect Donald Trump. To a significant degree, over-priced drugs are a problem partly caused by politicians. Congress allowed the regulatory regime at the U.S. Food and Drug Administration (FDA) to run rampant. But maybe the laws regulating drug discovery are beginning to change.
The House and Senate have passed the 21st Century Cures Act. Aside from $6 billion worth of mostly-unnecessary pork barrel spending, the Cures Act takes steps to reform the process of drug approval at the U.S. Food and Drug Administration (FDA).
A rational path to drug discovery is badly needed. Once a drug finally makes it through the approval process, it is guaranteed years of high monopoly prices due to the plethora of regulatory barriers that inhibit competition.
Consider this: When brand drugs face generic competition, the generic price falls precipitously. Research shows that when a brand drug faces generic competition from only one generic, the price of the competing generic is nearly as high (94 percent) as the brand name drug.
· By the time there are two competing generics, average generic price has fallen by half.
· By the time there are five competitors, the price is one-third.
· Moving from six to nine competing generics drops the price by three quarters compared to the brand drug.
Approving new competing “me-too” drugs also reduces drug prices for all drugs in the same class. Making it easier to bring "me-too" drugs to market can lower prices before drugs face generic competition while boosting access to advanced drug therapies. Moreover, boosting competition can also prevent some of the egregious price hikes we’ve witnessed in the past year.
Recommended
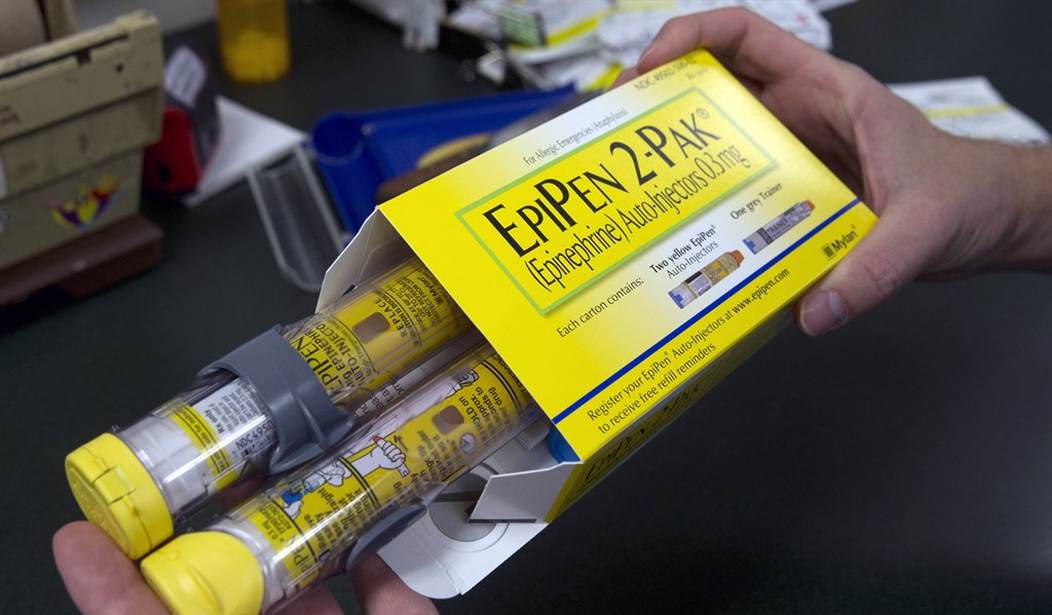
Consider the recent example of the EpiPen. It experienced price increases of about 450 percent during the past 10 years, despite being a 40-year old product. The EpiPen is a relatively simple drug delivery device (auto-injector) that administers roughly $1 worth of generic epinephrine. The current version of the EpiPen is under patent protection. But generic auto-injectors, used by diabetics to self-inject, sell for $30 to $40 apiece retail. Simple logic would suggest an epinephrine auto-injector should cost no more than $30+$1 or $40+$1. Yet, EpiPens sell for $300 apiece due to regulations that make it hard to bring competitive products to market. For example, the maker of generic auto-injectors cannot sell them pre-loaded with epinephrine.
Another problem that impedes drug development is that different divisions within the FDA use different standards for safe and effective. Whereas one division may merely look for a net benefit (benefits greater than risks); another division may require almost no risk and huge benefits. While one division may tolerate little risk, another may allow significant uncertainty if the drug is the first in its class.
Rising deductibles have made it more difficult for drug makers to disguise high prices by passing them on to insurers. In response to public scrutiny, drug companies began blaming the “middleman.” Middleman is an old boogeyman. If a retailer wants to convince consumers its products are cheaper, it claims “we’ve cut out the middleman.” In industry parlance, what drug makers call “the middlemen” is referred to as “the supply chain." Oddly enough, some drug makers cut out the middleman to allow them to charge higher prices. The strategy is designed to prevent drugstores from substituting a cheap generic in place of a high-priced drug for which a cheaper alternative exists.
Some drug makers also blame drug plans run by pharmaceutical benefit managers (PBMs) for higher prices. PBMs are the firms that administrate most consumers’ drug benefits. Insurers and employer plans hire PBMs to manage pharmacy benefits and adjudicate drug claims for plan members. PBMs clients are employers and insurers -- not drug makers. Drug plans do their best to hold down drug prices for plan sponsors - their clients - which occasionally makes them unpopular with drug makers and pharmacy owners.
So what does all this mean? Drug makers blaming the middleman for high drug prices is a straw man argument. Drug makers set prices for their products based on competing products and what the market will bear.
A better way to reign in high drug prices is to inject more competition into the drug market. New drugs would face numerous competitors if it didn’t cost $1 billion to bring new products to market. With more competition, high prices -- such as prescriptions that cost $2,000 per month -- would be impossible for drug makers to maintain. Competition works great in that regard. The 21st Century Cures Act would streamline the approval process for new drugs by allowing drug makers to take patient experiences into account and allow the FDA to consider aggregate anecdotal data as evidence. Rather than being limited to double-blind clinical trials that are rigid and costly, pharmaceutical companies could also track patient experiences and test out drugs’ effects using more evidence than just clinical trials.
But more needs to be done. Under current law, the FDA can already consider anecdotal data in addition to clinical trial data. But the agency has not implemented the 2012 provision allowing it to do so. The bureaucracy within the FDA is rigid and many of the investigators are set in their ways. A benefit of Congress providing guidance would be better prices for drugs Americans take.
























Join the conversation as a VIP Member