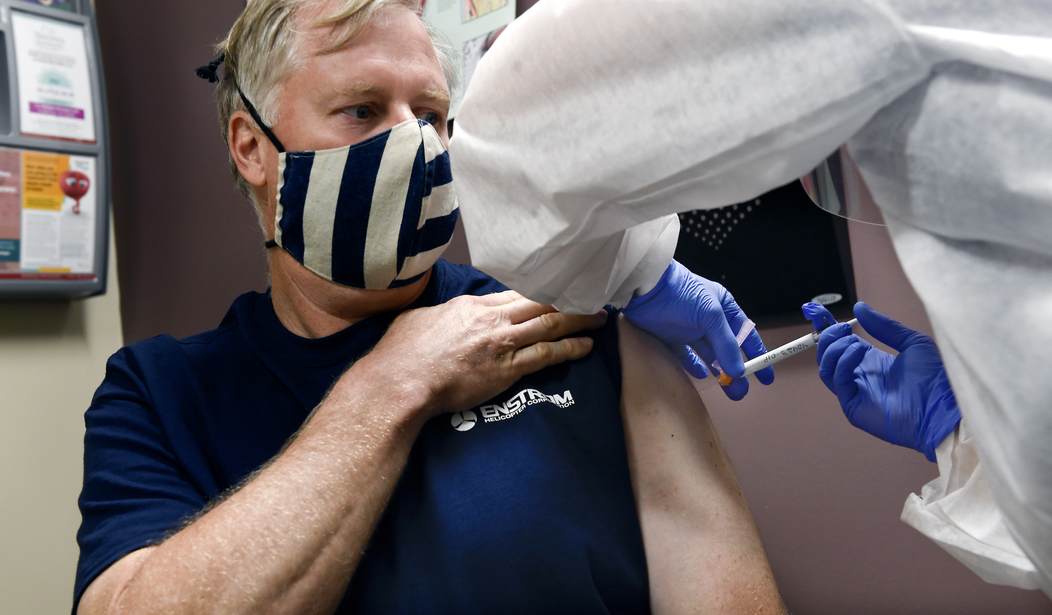
Major pharmaceutical company Moderna announced Monday stage three clinical trials of a Wuhan coronavirus vaccine have been 94.5 percent effective.
“This is a pivotal moment in the development of our COVID-19 vaccine candidate. Since early January, we have chased this virus with the intent to protect as many people around the world as possible. All along, we have known that each day matters. This positive interim analysis from our Phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease, including severe disease,” Moderna CEO Stéphane Bancel released in a statement. “This milestone is only possible because of the hard work and sacrifices of so many. I want to thank the thousands of participants in our Phase 1, Phase 2 and Phase 3 studies, and the staff at our clinical trial sites who have been on the front lines of the fight against the virus. They are an inspiration to us all. I want to thank the NIH, particularly NIAID, for their scientific leadership including through years of foundational research on potential pandemic threats at the Vaccine Research Center that led to the discovery of the best way to make Spike protein antigens that are being used in our vaccine and others’."
"I want to thank our partners at BARDA and Operation Warp Speed who have been instrumental to accelerating our progress to this point. Finally, I want to thank the Moderna team, our suppliers and our partners, for their tireless work across research, development and manufacturing of the vaccine. We look forward to the next milestones of submitting for an EUA in the U.S., and regulatory filings in countries around the world, while we continue to collect data on the safety and efficacy of the vaccine in the COVE study. We remain committed to and focused on doing our part to help end the COVID-19 pandemic,” Bancel continued.
The White House is celebrating the news.
????BIG NEWS! ????
— Kayleigh McEnany (@PressSec) November 16, 2020
Thanks to President @realDonaldTrump’s highly successful Operation Warp Speed, a second vaccine (Moderna) has shown high efficacy in Phase 3 clinical trial.
This news comes just after Pfizer likewise proved successful. Big wins for all??
The Moderna/ NIH vaccine is the 2nd candidate in a week to show strong efficacy in Phase 3 trials!
— Ivanka Trump (@IvankaTrump) November 16, 2020
Congratulations Moderna, @realDonaldTrump, the #OperationWarpSpeed team and all that made this historic breakthrough possible — it will help bring an end to this terrible pandemic! https://t.co/9S7ZH29nbL
Recommended
Last week Pfizer, also part of Operation Warp Speed, announced their version of the vaccine was 90 percent effective in clinical trials. President Trump and Operation Warp Speed leaders gave an update on vaccine progress and distribution plans from the White House Friday evening. It is expected 20 million doses will be distributed to the most vulnerable and high risk Americans by the end of December, with remaining distribution happening by April 2021.
LIVE: President @realDonaldTrump delivers an update on Operation Warp Speed https://t.co/6UQ0SZMsCQ
— The White House (@WhiteHouse) November 13, 2020
























Join the conversation as a VIP Member