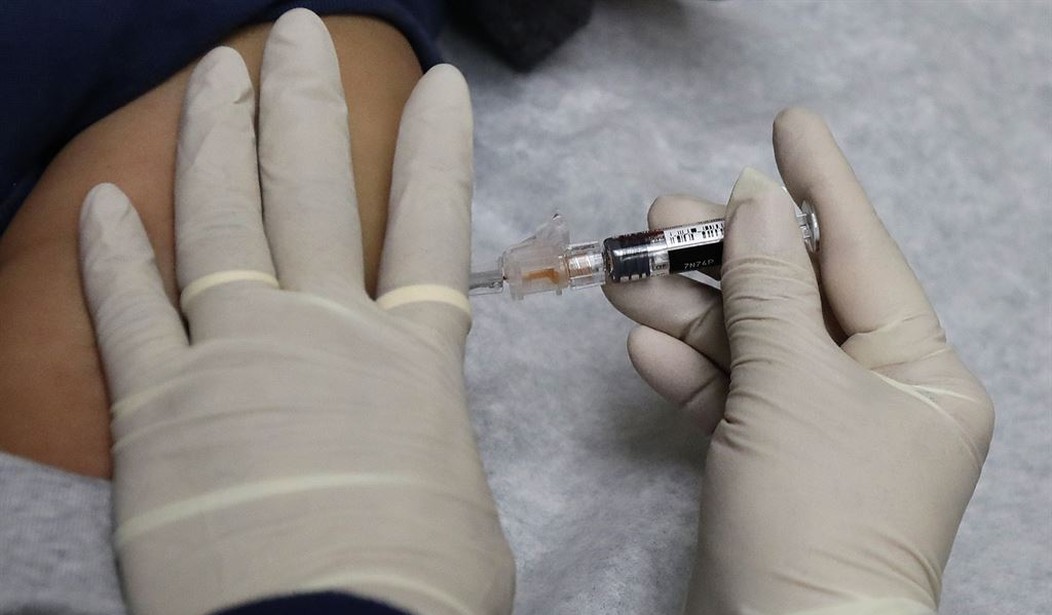
The first Wuhan coronavirus vaccine is showing promising results, meaning it's likely to begin a final clinical trial. Researchers say the vaccine boosted people's immune systems, which could be enough to protect against the virus.
The initial trial began in March with 45 young volunteers. Those original volunteers had neutralizing antibodies in their bloodstream, which are key to blocking infections. The research team told the New England Journal of Medicine the antibodies are similar to those who were infected and survived the virus.
“No matter how you slice this, this is good news,” Dr. Anthony Fauci, the head of the National Institute of Allergies and Infectious Diseases (NIAID) told the Associated Press.
The vaccine was developed by the National Institutes of Health and Moderna Inc., which has researchers from the NIAID working on the project.
Because of the positive results the original volunteers had, the next stage would mean testing the vaccine on 30,000 people. That will give researchers an idea of how effective the vaccine would be to protect the American public against the Wuhan coronavirus.
In the next phase, older adults and those with pre-existing conditions will be included. There will also be an emphasis on testing the vaccine on blacks and Latinos, which have also been heavily ravaged by the virus.
Recommended
“This is an essential building block that is needed to move forward with the trials that could actually determine whether the vaccine does protect against infection,” the study's leader, Dr. Lisa Jackson of the Kaiser Permanente Washington Research Institute in Seattle, told the AP.
The vaccine comes in two doses, one month apart. No serious side effects have been reported. The majority of volunteers had typical flu-like reactions, which are common with other vaccines, including fatigue, headache, chills, fever and pain at the injection site.
Those who wish to sign up to be part of the trail are encouraged to do so.























Join the conversation as a VIP Member